Product Options and Specifications
Matching Your Assay Needs: Defibrinated Plasma and Standard Human Serum
Defibrinated Plasma
A Core Processed Plasma Option
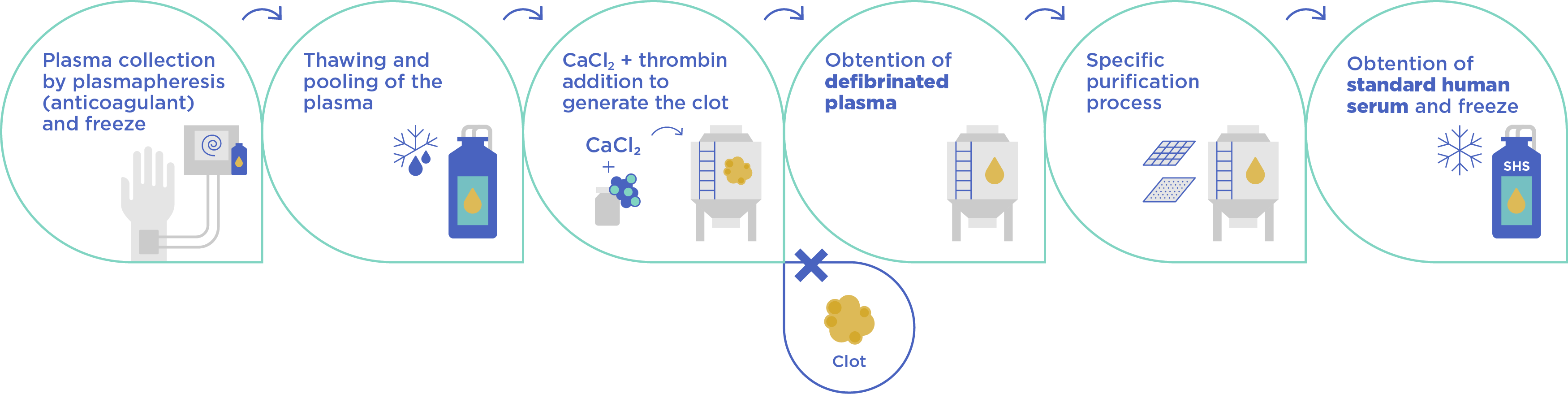
Defibrinated plasma forms the foundation of our processed human plasma offerings. It's produced by separating plasma from whole blood and then removing fibrinogen, a clotting factor. This defibrination process creates a more consistent and homogeneous sample compared to off-the-clot (OTC) serum.
Defibrinated plasma is a versatile option ideal for researchers needing a well-characterized and consistent processed plasma solution for their assays.
Standard Human Serum (SHS)
A Highly Purified Processed Plasma Option
Standard human serum (SHS) represents a further refinement of defibrinated plasma, offering the highest level of purity among our processed plasma options. It presents a reliable replacement for off-the-clot (OTC) serum.
SHS is produced by subjecting defibrinated plasma to a specialized high purification process that significantly lowers citrate levels from approximately 60 mg/dL to an average of 3 mg/dL, which is within the physiological range values.
SHS Advantages:
- Enhanced performance: Our high purification process elevates the effectiveness of standard defibrinated plasma by reducing citrate concentration to physiological levels.
- Increased availability: Compared to OTC serum, SHS offers greater availability due to its production through plasmapheresis.
- Customization options: We offer customization possibilities for SHS to meet your specific research needs.
SHS is the optimal choice for researchers requiring a high level of purity, consistency, and minimal interference for their assays. Its exceptional characteristics make it ideal for sensitive assays and research demanding the most reliable data.