Our Principles
Our business is grounded on the following attributes:
Grifols Bio Supplies business unit is a leader in human biological materials collected from our own network of blood and plasma centers to be used by pharmaceutical and biotechnology companies for drug manufacturing, the life-science industry for research, and in vitro diagnostic companies for the production of reagents and controls.
Grifols is a global healthcare company committed to improving people’s health and well-being. Our more than 24,000 employees in more than 30 countries and regions develop, produce, and market plasma-derived medicines as well as innovative biotech and diagnostic solutions in more than 110 countries.
Our business is grounded on the following attributes:

Safety
Source control of all materials is directly linked to safety

Excellence
We provide ethical, consent-based human biological material from the world's largest plasma collection network

Commitment
Our commitment to our customers inspires us to maintain the highest standards of quality in our products

Legacy of Innovation
Our on-going innovation has helped shape industry standards in the plasma sector
Grifols Bio Supplies products are ideally suited for further pharmaceutical manufacturing due to the high degree of regulatory support provided.
Immunoglobulin is used in a range of pharmaceutical applications, and it is increasingly being utilized in the cell and gene therapy field. Grifols immunoglobulin is manufactured from plasma originating from our own US and EU plasmapheresis centers, conforming to cGMPs, IQPP of the Plasma Protein Therapeutic Association (PPTA) and fulfilling additional requirements set by Grifols. Grifols immunoglobulin production processes and facilities are certified according to the US FDA and EU regulations and are licensed according to both USP and EP requirements.
Human serum albumin is a highly versatile raw material for further manufacturing. Human serum albumin is used in cell therapy manufacturing, cell culture media and as an excipient for a variety of therapeutic products. Grifols Human Albumin is a pasteurised, high-purity albumin solution which is licensed according to both USP and EP requirements. Grifols albumin production processes and facilities are certified according to the US FDA and EU regulations.
Several of the BioPharma products are provided with a very high level of regulatory support meaning that certain products can be utilised as both a raw material and as an excipient.
Consistent attributes qualified Grifols Bio Supplies as your supplier of choice for the procurement of human-derived blood materials.
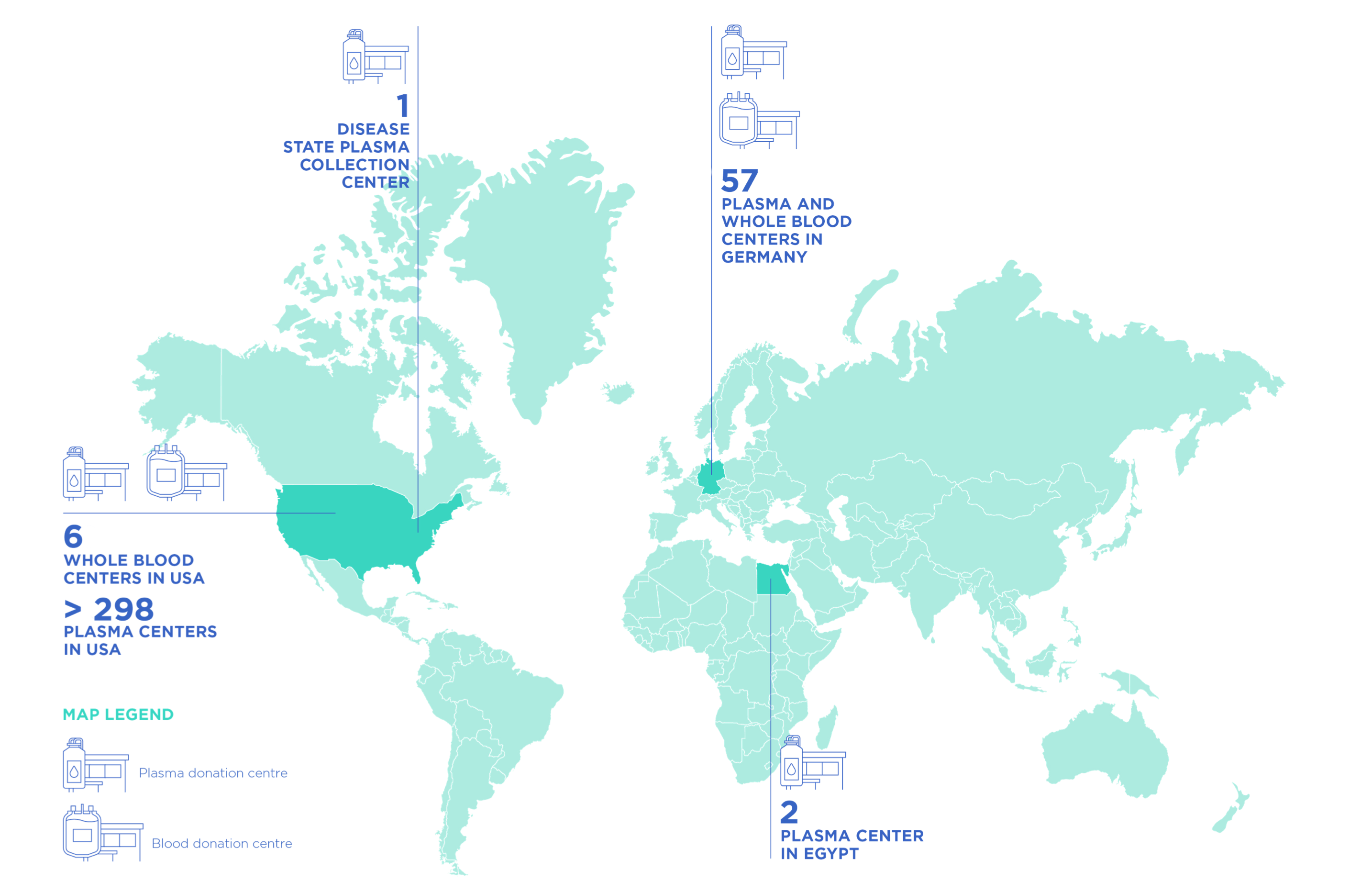
Grifols has the largest worldwide plasma donor center network with more than 10 million annual donations.

High Quality
All of our collection centers located in the USA are FDA-licensed facilities. All of our collection centers located in Germany are licensed by the respective federal state authority.

Product Customization
Customizable product capabilities to align with the customer needs and specifications: anticoagulants, processing, packaging, storage, temperature.

Viral Donor Testing
All units are tested according to local regulations to ensure they are negative/non-reactive for infectious diseases. Additional tests can be provided upon customer demand.

Donor Data
We collect the following data for all donors in which we collect human-derived blood materials from: age, race, gender, blood group, smoker/non-smoker, health conditions and other factors.
The information contained in these webpages are intended for a professional audience of pharmaceutical and in vitro diagnostic manufacturing personnel. All products are intended for research and development and manufacturing usage.
Availability of the products are subjected to certain regions. For more information, please contact us
Contact a Bio Supplies representative today to get more information.